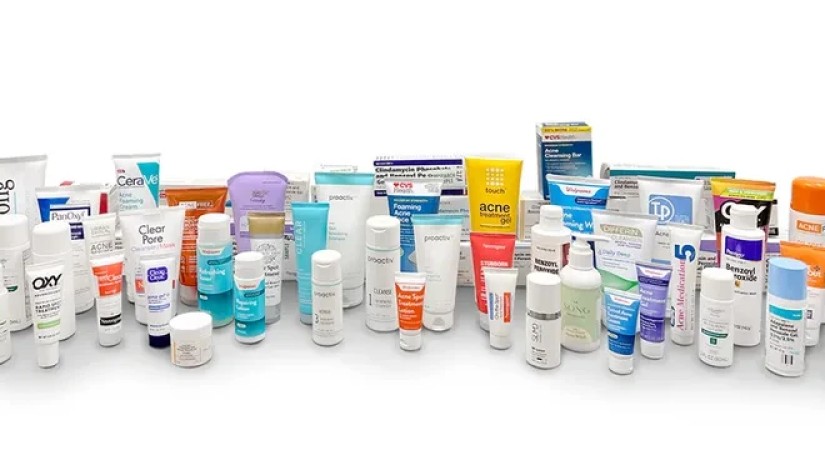
An independent laboratory is calling on federal health officials to recall popular benzoyl peroxide acne treatment products after discovering that they contain high levels of a chemical linked to cancer.
Valisure on Wednesday said that substantial amounts of benzene, a human carcinogen, can form in prescription and over-the-counter benzoyl peroxide acne treatment products that are handled or stored at certain temperatures.
The lab tested 66 benzoyl peroxide acne treatment products, including creams, lotions, gels, washes, liquids and bars, and discovered that popular brands such as Clearasil, Proactiv, Target's Up & Up brand and Clinique can form benzene "at hundreds of times the conditional FDA limit," Valisure co-founder David Light said in a statement.
The current evidence from the recent tests suggests that this problem "applies broadly to benzoyl peroxide products that are currently on the market," according to the lab.
In extreme cases, the Food and Drug Administration (FDA) will allow up to 2 parts per million of benzene inside a drug product that the FDA regulates. However, the tests show that benzene inside the benzoyl peroxide products is more than 800 times that when stored at 122 degrees, and up to nine times that when the products are sitting on the shelf.
The high levels of the chemical were not only detected inside the products, but also in the air outside of benzoyl peroxide products, "showing that benzene can leak out of some product packages and pose a potential inhalation risk," Valisure said in a notice.
The EPA has an air limit for benzene. Per the agency, the elevated cancer risk at the standard regulatory level starts at 0.4 parts per billion (ppb). When Valisure calculated benzene air results, in certain cases, it was 1,270 times the EPA threshold.
In a petition to the FDA, filed Tuesday, Valisure requested an investigation and market withdrawal of benzoyl peroxide-containing products.
FOX Business has reached out to the FDA for comment.
Light said benzoyl peroxide’s benzene formation "is substantially different" than previous findings of benzene in other consumer products like sunscreens and hand sanitizers.
According to the FDA's website, benzene is used in the production of a wide range of industrial products, from dyes and detergents to some plastics. It is also released into the air through cigarette smoke and emissions from automobiles as well as burning coal and oil.
However, in recent years, several products such as dry shampoos, hand sanitizers and sunscreens have been recalled for having excess levels of the chemical, posing a health risk to consumers.
The FDA said the health consequences of exposure to benzene can "depend on the amount, route, and length of time of exposure" as well as someone's age and any preexisting medical conditions.
Long-term exposure to the chemical through inhalation, oral intake and skin absorption may result in cancers such as leukemia and other blood disorders, the FDA said.
A spokesperson from Reckitt, which owns the Clearasil brand, said the company is "confident that all Clearasil products, when used and stored as directed on their labels, are safe" and that it works "closely with regulators around the world to ensure our products are safe and effective for their intended use."
"Clearasil products and their ingredients are stable over the storage conditions described on their packaging which represent all reasonable and foreseeable conditions," the company said.
The company also asserted that the findings presented by the independent lab "reflect unrealistic scenarios rather than real-world conditions."
Source: Fox Business