On the day Britain began immunizing its population with a Covid-19 shot developed by Germany’s BioNTech SE and the U.S.’s Pfizer Inc., a peer-reviewed study of the U.K.’s homegrown vaccine left some key questions unanswered, - Bloomberg reports.
While trial results published Tuesday in The Lancet found that a vaccine developed by the University of Oxford and AstraZeneca Plc is safe and effective, more analysis will be needed to see how well it works in people over 55, among those at higher risk from the pandemic.
Because older adults were recruited to the studies later than younger ones, “they’ve had less time for cases to accrue in those age groups and for us to be able to measure an efficacy signal,” said Andrew Pollard, lead investigator on the Oxford trial. “The evidence we have so far on the immune response very much suggests that it’s likely to be similar levels of protection across the ages.”
The Pfizer-BioNTech shot, approved in Britain last week, could be cleared in the U.S. as soon as this week and the European Union by the end of the year as governments race to overcome a contagion that has taken more than 1.5 million lives and devastated economies. The Astra-Oxford shot looks less effective than that vaccine and another from Moderna Inc., which each showed 95% efficacy in trials, but is expected to be cheaper and easier to deploy.
The Lancet report provides the first peer-reviewed data from any of the leading vaccines.
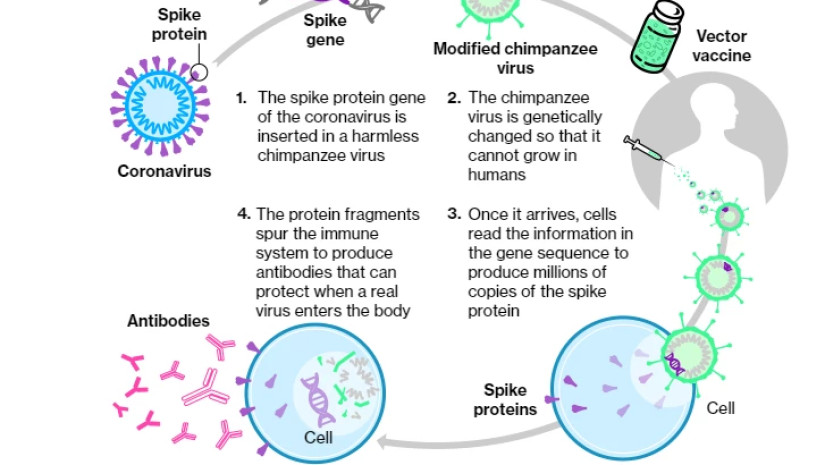
How the Oxford-AstraZeneca Vaccine Works
The viral vector vaccine uses a harmless virus to transport genetic material which triggers an immune response to the coronavirus.
The initial data from Astra and Oxford last month appeared positive but raised concern over how much protection the shot would offer after the trials produced two different results from two dosing regimens. The partners said their vaccine was 90% effective when a half-dose was given before a full-dose booster, and that two full doses showed an efficacy of 62%.
It later emerged the lower dose was the result of a manufacturing mistake and only tested in a younger group. Astra Chief Executive Officer Pascal Soriot said in an interview last month the company would set up an additional, probably global, trial to verify the 90% result. On Tuesday, the company said it’s still deciding whether to hold an additional study, while Pollard said Oxford had no plans for an extra U.K. trial at present. A separate study is underway in the U.S.