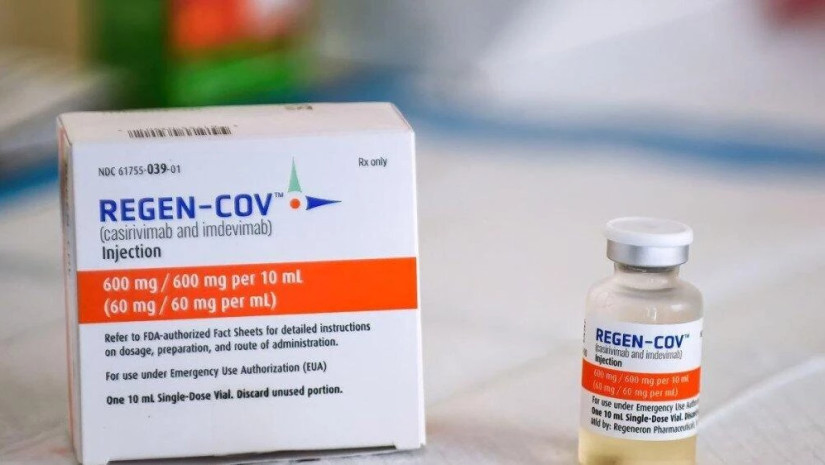
The WHO announced Friday it was recommending a cocktail of monoclonal antibodies manufactured by the US biotech firm Regeneron and marketed by Swiss pharmaceutical giant Roche to treat COVID-19 in certain patients.
The combination of two drugs, the monoclonal antibodies casirivimab and indevimab, bind to the SARS-CoV-2 spike protein, neutralizing the virus and preventing it from infecting more cells. It is only the third treatment to be approved for treatment of COVID-19 by the WHO, Deutsche Welle reports.
The cocktail, marketed as REGEN-COV in the US and Ronapreve elsewhere, was recommended only for certain patients, namely those who had a previous illness or are at risk of falling seriously ill from COVID-19 and facing time in intensive care.
"For all other COVID-19 patients, any benefits of this antibody treatment are unlikely to be meaningful," the WHO said.
Publishing in the British Journal of Medicine, the UN health body cited data from a large British study and three trials are not peer reviewed yet. The drug cocktail gained attention worldwide when physicians at Walter Reed Medical Center gave it to then US President Donald Trump when he had COVID-19.
The WHO was joined by Doctors Without Borders in expressing concerns about the high cost of the treatment.
Global public health will depend on pharmaceutical manufacturers and governments working together to bring costs down, especially in the developing world, the UN health agency said, adding other agencies were involved in talks with Roche.
Doctors Without Borders said Regeneron has filed patent applications in at least 11 impoverished nations.
Japan was first to authorize its use last July. The EU, US, India, Switzerland and Canada have already approved the drug combination for emergency use.